I’ve got two problems. First, my ornamental garden pond is filled with algae. In the past, I have simply emptied it out and refilled, but I’m tired of doing that, and I also suspect there are some tadpoles growing in the pond and I’d like to let them do their thing, if possible.
Second, COVID has shut down summer research at my school, and I’m in need of a science project to keep my mind in the game. Since I work mostly with instrumentation design, I brought much of my lab home with me during the transition to on-line learning this semester. One of the instruments I brought back was a Vernier Labquest with the SpectroVis Plus spectrophotometer/fluorimeter.
With most of New York having recently entered Phase II of our return to normal – whatever normal will look like – my wife and I have been spending our stimulus checks at the local garden supply store. One of their products is an algaecide that “works fast”. That got me thinking; I don’t know “fast” means in the algaecide’s promotional language, but I need to clean up this pond and I can use the spectrometer to help me measure how fast is fast.
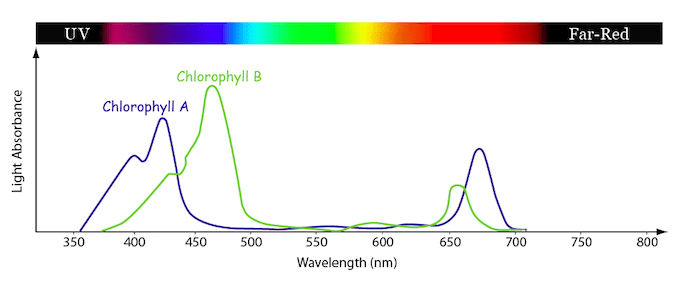
Algae contains chlorophyll, which has an absorption spectrum that looks something like this:
The Vernier LabQuest has a cool feature where it can be connected to my home internet making it easy for me to view and transfer data that has been collected on the device. The web interface is bare bones so the graphs are not publication quality; however that’s not what I’m after here. Below is a visible spectrum of the pond water.

The maximum in the red region is around 680 nm which is more consistent with chlorophyll A. The broad peak at 432 supports this assignment. There is a shoulder at 500 nm and a small feature at 625, which suggests to me that some chlorophyll B is also present. The near-IR feature around 920 is a mystery. The green line in the spectrum is a blank obtained by filling the cuvette with tap water (no distilled water tap in my house, sadly). It shows that the pond-water spectrum is offset quite a bit. In addition to being filled with algae, the pond water is fairly turbid, and the increased absorbance observed in the spectrum is likely due to this turbidity.
I left the turbidity sensor in my lab, so if I get really bored, I may need to walk over to the college and pick it up for this summer science project.
The spectravis also allows me to acquire fluorescence spectra at two different wavelengths. I opted for 405 nm for no particular reason and obtained the following spectrum.

The large peak at 405 nm is from the excitation source. I’m using a plastic cuvette which is scattering much of the excitation light and I suspect that is why this peak is so intense. The smaller peak at 690 nm has an intensity of 0.046 units and is likely due to chlorophyll. We wouldn’t expect a strong signal from chlorophyll B under these conditions since the excitation wavelength that I selected would not be absorbed by chlorophyll B to a great extent.
With some background spectra collected, I added the algaecide. The instructions recommend 5 mL per 50 gallons. I estimate from geometry that my pond cannot be more than about 165 gallons so I added 15 mL. Now it’s time to let the chemistry do its job. I’ll be back to monitor it later on today.

Whoa. If it’s 5 ml per 50 gallons and you have 165 gallons then you should add about16.5 ml. Not 150 ml. What did I miss?
Good catch! That’s a typo. I added 15 mL, not 150 mL. I guess even my blog posts need peer review :-).